Abstract
Background.
Idiopathic PRCA and secondary PRCA not responding to the treatment of the underlying diseases are generally treated by immunosuppressive therapy. Because of the rarity of this disease, there are few reports regarding the long-term outcome following immunosuppression. We previously conducted the nationwide study for chronic PRCA in Japan that had been diagnosed between 1990 and 2006 across 109 institutions, and collected the data on a total of 185 patients (PRCA2004/2006 study). This study revealed that poor response to induction therapy and relapse of anemia were associated with death. There was no significant difference in survival between idiopathic and secondary PRCA in the PRCA2004/2006 study cohort.
Objective.
In order to identify the adverse risk factors for survival in acquired PRCA following immunosuppression in a prospective way, we have conducted another nationwide cohort study for acquired PRCA in adults (PRCA2016 study). The primary endpoint is the overall survival (OS) and the secondary endpoints include efficacy of immunosuppression, causes of treatment failure and survival times with iron-chelation therapy.
Methods.
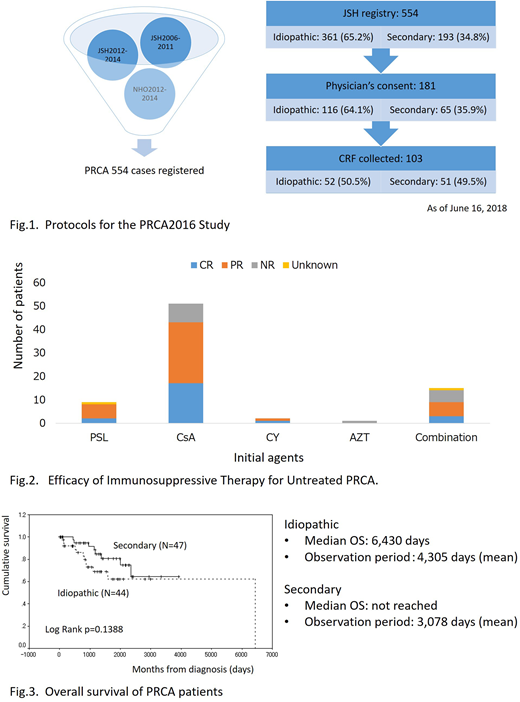
This is designed as a prospective longitudinal observational study. Between 2006 and 2014, 554 PRCA patients were registered in the hematological disorder registry managed by the Japanese Society of Hematology. We sent the first questionnaires to the physicians asking for collaboration with this study and the responses showed the potential size of cohort was 181 patients (Fig. 1). We then sent the second questionnaires to collect data regarding underlying disease, laboratory findings including CBC and leukocyte differentials, results of bone marrow examination, immunological and cytogenetics, efficacy of immunosuppression, iron-chelation therapy in refractory cases and outcome. The review boards of participating institutions and the ethical committee of the JSH approved this study and informed consent was obtained from the patients.
Results.
As of June 16, 2018, 103 patients were registered in this study. Fifty-two patients were classified as having idiopathic PRCA and 51 patients as having secondary PRCA, including 16 thymoma- and 5 large granular lymphocyte (LGL) leukemia-associated PRCA. Seventy-one patients were treated with immunosuppressive agents. The response rates of cyclosporine, predonisolone, cyclophosphamide, azathioprine and combination with more than one immunosuppressants were 84% (43/51), 89% (8/9), 100% (2/2), 0% (0/1), 60% (9/15), respectively (Fig. 2). The Kaplan-Meyer estimates of the survival revealed that the estimated median survival time in idiopathic PRCA was 6,430 days and that in secondary PRCA has not yet been reached (Fig. 3). Survival time was not significantly different between the two subtypes of PRCA. Twenty-two deaths were reported and the major causes of death were infection and heart failure.
Discussion/Conclusion.
The most common subtypes of chronic PRCA in Japan were idiopathic, thymoma-associated and LGL leukemia-associated PRCA and this result was consistent with our observation in the PRCA2004/2006 study. Cyclosporine and prednisolone were frequently chosen for the induction therapy resulting in favorable responses. Preferential use of these two agents might reflect the physician's concern about the toxicity of the cytotoxic drugs. The median survival time of idiopathic PRCA was quite similar to the results in the previous cohort study. No significant difference in survival between idiopathic and secondary PRCA has been confirmed by the present study. These two cohort studies clearly indicate that novel diagnostic and predictive biomarkers should be developed to segregate the good and poor responders to immunosuppressive therapy in PRCA.
Nakao:Novartis: Honoraria; Kyowa Hakko Kirin Co., Ltd.: Honoraria; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. Yonemura:Alexion Pharma: Honoraria, Research Funding. Matsuda:GlaxoSmithKline K.K.: Honoraria; Novartis Pharma K. K.: Honoraria; Chugai Pharmaceutical Co, Ltd.: Honoraria; Kyowa Hakko Kirin Co, Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Celgene Corporation: Honoraria; Alexion Pharmaceuticals, Inc.: Honoraria; Sanofi K.K.: Honoraria; Beckman Coulter K.K.: Honoraria. Kurokawa:Otsuka Pharmaceutical: Research Funding; Astellas Pharma: Research Funding; MSD: Honoraria, Research Funding; Pfizer: Research Funding; Eizai: Research Funding; Ono Pharmaceutical: Honoraria, Research Funding; Sumitomo Dainippon Pharma: Research Funding; Teijin Pharma: Research Funding; Nippon Sinyaku: Honoraria, Research Funding; Takeda Pharmaceutical: Research Funding; Chugai Pharmaceutical: Research Funding; Kyowa Hakko Kirin: Honoraria, Research Funding. Arai:Novartis: Research Funding. Mitani:Kyowa Hakko Kirin Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; Bristol-Myesr Squibb: Research Funding, Speakers Bureau; Celgene: Speakers Bureau; Chugai: Research Funding; Astellas: Research Funding; Sumitomo Dainippon: Research Funding; Novartis: Research Funding; Toyama Chemical: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal